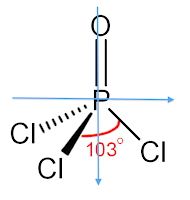
Phosphoryl Chloride
Phosphoryl Chloride (POCl3)
Phosphoryl Chloride, more commonly known as phosphorus oxychloride, is a colorless liquid. It hydrolyses into phosphoric acid in the presence of moist air. It is produced industrially in large quantities and is primarily used to make phosphate esters.
Polarity
The molecule POCl3 is a polar molecule because there is an uneven distribution of electrons throughout the molecule. This can be easily determined by attempting to draw a line of symmetry through the molecule. If there are one or fewer lines of symmetry that can be drawn through the molecule, then the molecule is polar. No lines of symmetry can be drawn through this molecule, determining that the molecule is polar. This lewis structure below shows the molecule's lack of a line of symmetry.
The bonds on the molecule POCl3 are covalent. The bond of phosphorus to oxygen is slightly-moderately covalent. The bonds of phosphorus to chlorine are moderately covalent.
A bond of an atom is either covalent or ionic. This is determined by the difference of the two element's electronegativity values. If the result is between 0 and 1.6, then the bond is covalent. If it is between 1.8 and 3.0, the bond is ionic. However if the bond is exactly 1.7, then the bond is neutral.
For this molecule, oxygen has an electronegativity value of 3.5 and phosphorus, 2.2. When subtracted the result is 1.3, making the bond slightly-moderately covalent. Chlorine has an electronegativity value of 3.2. When the electronegativity value of phosphorus (2.2) is subtracted from the electronegativity value of chlorine (3.2), the result is 1.0. This makes the bond moderately covalent.
The bonds on the molecule POCl3 are covalent. The bond of phosphorus to oxygen is slightly-moderately covalent. The bonds of phosphorus to chlorine are moderately covalent.
A bond of an atom is either covalent or ionic. This is determined by the difference of the two element's electronegativity values. If the result is between 0 and 1.6, then the bond is covalent. If it is between 1.8 and 3.0, the bond is ionic. However if the bond is exactly 1.7, then the bond is neutral.
For this molecule, oxygen has an electronegativity value of 3.5 and phosphorus, 2.2. When subtracted the result is 1.3, making the bond slightly-moderately covalent. Chlorine has an electronegativity value of 3.2. When the electronegativity value of phosphorus (2.2) is subtracted from the electronegativity value of chlorine (3.2), the result is 1.0. This makes the bond moderately covalent.
Forces of Attraction
Intermolecular Forces of Attraction are forces that exist between two molecules that cause their attraction to each other. Every molecule has at least one force of attraction. There are three forces of intermolecular attraction. These are: London Dispersion Forces, Dipole-Dipole Attraction, and Hydrogen Bonding, and are defined as follows:
London Dispersion Forces: Because electrons are constantly in motion, one end of a (non)polar molecule is temporarily either positive or negative. Because of these temporary dipoles, two (non)polar molecules have a very weak force of attraction between them.
Dipole-Dipole Attraction: An electrostatic attraction between the positive or negative end of one molecule and the oppositely charged end of another dipole.
Hydrogen Bonding: A special case of dipole-dipole attraction, hydrogen bonding is a temporary covalent bond between two dipoles containing hydrogen and oxygen, fluoride, or nitrogen.
Above is a lewis structure model of the attraction between two molecules of POCl3. This attraction can occur by either London Dispersion Forces or Dipole-Dipole Attraction. It can not, however, occur by the force of hydrogen bonding because the molecule does not contain any hydrogen to bond to the oxygen at the negative end of the molecule.
London Dispersion Forces: Because electrons are constantly in motion, one end of a (non)polar molecule is temporarily either positive or negative. Because of these temporary dipoles, two (non)polar molecules have a very weak force of attraction between them.
Dipole-Dipole Attraction: An electrostatic attraction between the positive or negative end of one molecule and the oppositely charged end of another dipole.
Hydrogen Bonding: A special case of dipole-dipole attraction, hydrogen bonding is a temporary covalent bond between two dipoles containing hydrogen and oxygen, fluoride, or nitrogen.
Above is a lewis structure model of the attraction between two molecules of POCl3. This attraction can occur by either London Dispersion Forces or Dipole-Dipole Attraction. It can not, however, occur by the force of hydrogen bonding because the molecule does not contain any hydrogen to bond to the oxygen at the negative end of the molecule.
Subscribe to:
Comments (Atom)