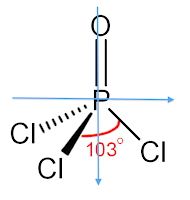
The molecule POCl3 is a polar molecule because there is an uneven distribution of electrons throughout the molecule. This can be easily determined by attempting to draw a line of symmetry through the molecule. If there are one or fewer lines of symmetry that can be drawn through the molecule, then the molecule is polar. No lines of symmetry can be drawn through this molecule, determining that the molecule is polar. This lewis structure below shows the molecule's lack of a line of symmetry.
The bonds on the molecule POCl3 are covalent. The bond of phosphorus to oxygen is slightly-moderately covalent. The bonds of phosphorus to chlorine are moderately covalent.
A bond of an atom is either covalent or ionic. This is determined by the difference of the two element's electronegativity values. If the result is between 0 and 1.6, then the bond is covalent. If it is between 1.8 and 3.0, the bond is ionic. However if the bond is exactly 1.7, then the bond is neutral.
For this molecule, oxygen has an electronegativity value of 3.5 and phosphorus, 2.2. When subtracted the result is 1.3, making the bond slightly-moderately covalent. Chlorine has an electronegativity value of 3.2. When the electronegativity value of phosphorus (2.2) is subtracted from the electronegativity value of chlorine (3.2), the result is 1.0. This makes the bond moderately covalent.
No comments:
Post a Comment